Abstract
Background:BCR-ABL-negative MPNs arise from mutations that dysregulate JAK-STAT signalling. SH2B3 encodes LNK, a key negative regulator of this pathway [1]. Somatic loss of function mutations and missense variants in SH2B3 occur in MPNs [2].
Aim: To validate missense germline SH2B3 variants as a cause for cytoses by characterising affected families, and murine and cell line models.
Methods: A comprehensive myeloid NGS panel of 46 genes was employed to screen >300 patients with MPNs [3]. The panel is used up front in most patients with JAK2-negative PV, ET, and MF at our institution. Clinical history, blood and bone marrow examination was obtained from individuals with an SH2B3 variant. Buccal DNA was used to confirm or refute germline variants. Biochemical and structural studies were undertaken using recombinant mutant LNK-SH2 domains, as described [4]. Cell line models were generated by CRISPR/Cas9 gene editing. A mouse model harbouring a missense mutation in Sh2b3, E367K (=E395K in human), was developed using gene editing in oocytes. Blood, spleen and bone marrow from mice was examined by histology, immunohistochemistry, and flow cytometry. Western blots and qRT-PCR was performed on platelets. Bone marrow transplantation (BMT) was undertaken in syngeneic CD45.1 mice to determine whether disease was cell or non-cell autonomous. Statistical analysis was performed using a Mann-Whitney test; a P value of <.05 was considered significant. The UK biobank was interrogated to determine the statistical association between specific variants and blood cell counts.
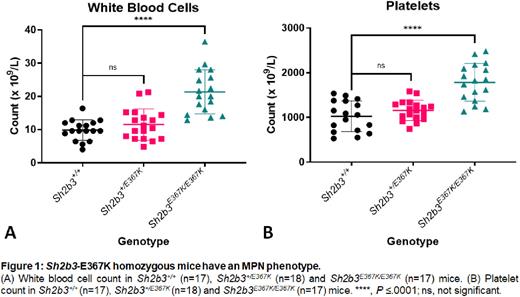
Results: Variants in SH2B3 were detected in 2.5% of all patients referred for NGS investigation of cytoses; the majority were missense variants within the SH2 domain. Most were present as isolated variants in triple negative ET or erythrocytosis, but some occurred in combination with driver mutations in advanced MPNs. Initially most variants were curated as variants of uncertain significance due to high frequencies in normal population databases, and limited evidence of pathogenicity; they were upgraded to 'likely pathogenic' based on this study. Cytoses were inherited in three families with SH2B3 variants. In two families, the probands were either homozygous for E395K in the blood via uniparental disomy of a heterozygous germline variant, or compound heterozygous for E395K and E400K. The affected members had clinical and bone marrow features of ET whilst heterozygous family members were unaffected. The proband in the third family had a heterozygous mutation (T404A) and advanced MF by two years of age, suggesting T404A is more damaging to function. Analysis of recent WES data from the UK Biobank demonstrated a strong association between platelet count and E395K (P value 1.45e-57) or E400K (P value 4.59e-113). There was also a significant association between these variants and leukocyte count and RBC distribution width (RDW). These variants have a combined allele frequency ~1:3,000. The T404A variant is rare in population databases. Binding of missense variants to pY813 of JAK2 indicated variable reductions in affinities. Cell line experiments demonstrated defective down regulation of MPL-JAK2-STAT3 signalling by missense variants compared with the wild type LNK. Sh2b3-E367K homozygous mice develop an MPN (Fig 1) with cytoses, splenomegaly and MF, as determined by reticulin staining. The phenotype is milder than that in Sh2b3-knockout mice [5]. Platelets from Sh2b3-E367K homozygous mice have increased pSTAT3 levels, consistent with hyperactive MPL-JAK2-STAT3 signalling. The cell-intrinsic nature of the phenotype was confirmed by BMT.
Conclusion: Germline missense variants in SH2B3 cause cytoses and sometimes significant MPN. Many cases are likely to have been missed up until now because SH2B3 is not present in all NGS panels, and because common variants have likely been curated as benign. The phenotypes are variable due to variable loss of binding to pY813 of JAK2, and variable exaggeration of JAK2-STAT signalling. The Sh2b3-E367K mouse model is a valuable tool for testing drugs that might alter the natural history of progression of ET to MF.
References: 1. Velazquez, L., et al., J Exp Med, 2002. 195(12): p. 1599-611.
2. Morris, R., et al., Pharmaceuticals, 2021. 15(1).
3. Magor, G.W., et al., J Mol Diagn, 2016. 18(5): p. 707-718.
4. Morris, R., et al., Nat Commun, 2021. 12(1): p. 6110.
5. Bersenev, A., et al., J Clin Invest, 2010. 120(6): p. 2058-69.
Disclosures
Grigg:Novartis: Membership on an entity's Board of Directors or advisory committees. Perkins:Sierra Oncology: Honoraria, Other: Support for present manuscript; Celgene: Other: Support for meetings and/or travel; Abbvie: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal